The history of Chemotherapeutic agents:
- Bacteria were first identified in the 1670s by van Leeuwenhoek, following his invention of the microscope.
- This appreciation followed the elegant experiments carried out by the French scientist Pasteur, The possibility that these microorganisms might be responsible for disease began to take hold.
- During that latter half of the nineteenth century, scientists such as Koch were able to identify the microorganisms responsible for diseases such as tuberculosis, cholera, and typhoid.
- Methods such as vaccination for fighting infections were studied. Research was also carried out to try and find effective antibacterial agents or antibiotics.
- However, the scientist who can lay claim to be the father of chemotherapy—the use of chemicals against infection—was Paul Ehrlich.
- Ehrlich spent much of his career studying histology, then immunochemistry, and won a Nobel prize for his contributions to immunology.
- By 1910, Ehrlich had successfully developed the first example of a purely synthetic antimicrobial drug.
- This was the arsenic-containing compound “Salvarsan.”
- Although it was not effective against a wide range of bacterial infections, it did prove effective against the protozoal disease sleeping sickness (trypanosomiasis), and the spirochaete disease of syphilis.
- The drug was used until 1945 when it was replaced by penicillin.
- Prolavine is a yellow-colored aminoacridine structure which is particularly effective against bacterial infections in deep surface wounds, and was used to great effect during the Second World War.
- Despite the success of this drug, it was not effective against bacterial infections in the bloodstream and there was still an urgent need for agents which would fight these infections.
- This need was answered in 1935 with the discovery that a red dye called Prontosil was effective against streptococci infections in vivo.
- Prontosil was eventually recognized as being a prodrug for a new class of antibacterial agents—the sulfa drugs (sulfonamides).
- The discovery of these drugs was a real breakthrough, since they represented the first drugs to be effective against bacterial infections carried in the bloodstream.
- They were the only effective drugs until penicillin became available in the early 1940s.
Sulfonamides:
Chemical Structure of Sulfonamides:
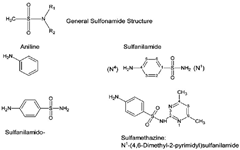
Nomenclature of Sulfonamides:
-
Sulfonamides is a generic term that includes following cases,
-
Antibacterial compounds that are “aniline substituted Sulfonamides: Sulfanilamides e.g. Sulfamethoxazole [4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide].

-
Prodrugs that produce active sulfanilamides e.g. Succinyl sulfathiazole.
-
Non aniline Sulfonamides e.g. Mefenide.

Structure Activity Relationship (SAR) of Sulfonamides:
-
The synthesis of large number of Sulfonamide analogues gave us following conclusions,

- The p-amino group is essential for activity and must be unsubstituted (i.e. R = H).
- The only exception is when R = acyl (i.e. amides).
- The amides themselves are inactive but can be metabolized in the body to regenerate the active compound.
- Thus amides can be used as sulfonamide prodrugs.
3. The aromatic ring must be para-substituted only.
4. The sulfonamide nitrogen must be secondary.
5. R2 is the only site where substitution is allowed.
6. Substitution at R2 with heterocyclic rings and homocyclic rings produced active compounds.
7. R2 substitution changes compounds solubility, plasma protein binding in short pharmacokinetics.
8. These substitutions doesn't affect mechanism of action of the compounds.
Mechanism of Action of Sulfonamides:
- Bacteria needs “Folic acid” for their nucleic acid synthesis, for which they are largely dependent on their hosts.
- The broken/ injured host cells contains a chemical called as “Para Amino Benzoic Acid” (PABA).

- This PABA is utilized by bacteria as a starting material to convert it to “Folic acid” using a enzyme called as “Folate Synthtase”.
- Sulfonamides has structural similarity with PABA.


- The enzyme gets fooled and picks up Sulfonamide which results in stoppage of folic acid synthesis and hence stopping bacterial nucleic acid synthesis.
- As Sulfonamides stops growth of bacterias they are “Bacteriostatic” in action.
- The uptake of Sulfonamides and PABA by bacterial folate synthtase is dependent on concentration and the phenomenon is called as “Competitive Inhibition”.
- Due to competitive inhibition large concentration of PABA can displace Sulfonamides from enzyme sites as happens in case of “pus” rendering Sulfonamides useless in presence of pus.
Classification of Sulfonamides:
-
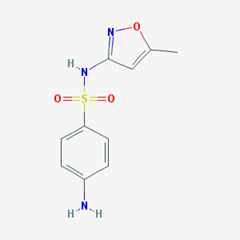
Sulfonamides with aniline N1 substitution:
- e.g Sulfamethoxazole. [4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide].
- Sulfisoxaole. [4-amino-N-(3,4-dimethyl-1,2-oxazol-5-yl)benzenesulfonamide].
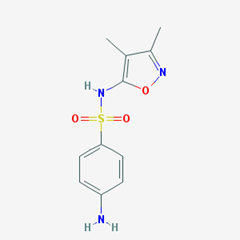
- Sulfadimidine. [4-amino-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide].

-
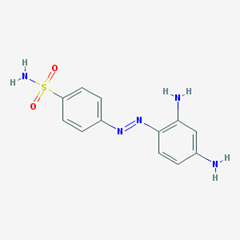
Sulfonamides with aniline N4 substitution:
- e.g. Prontosil. [4-[(2,4-diaminophenyl)diazenyl]benzenesulfonamide].
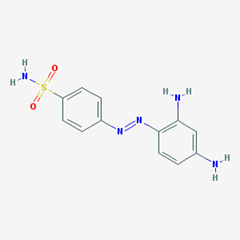
-
Sulfonamides with both N1 and N4 aniline substitution:
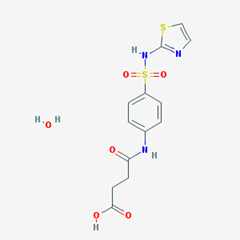
- e.g. Succinyl Sulfathiazole. [4-oxo-4-[4-(1,3-thiazol-2-ylsulfamoyl)anilino]butanoic acid].
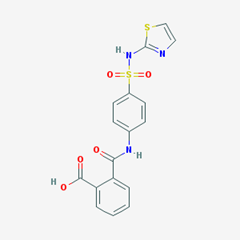
- Pthalyl Sulfathiazole. [
- 2-[[4-(1,3-thiazol-2-ylsulfamoyl)phenyl]carbamoyl]benzoic acid].
-
Sulfonamides without aniline ring:
- e.g. Mefenide acetate. [
- acetic acid;4-(aminomethyl)benzenesulfonamide]
Applications of Sulfonamides:
- The sulfa drugs presently have the following applications in medicine:
- Treatment of Urinary Tract Infections (UTI).
- Treatment of Eye Infections. (Sulfacetamide Sodium)
- Treatment of mucosal membrane infections. (Soft tissue infection: Sulfamethoxazole).
- Treatment of burns. (Silver Sulfadiazine)
- Treatment of ulcerative colitis. (Succinyl and Pthalyl Sulfathiazole).
- To counter various infections associated with Acquired Immuno Deficiency Syndrome (AIDS).
Adverse Effects / Side effects of Sulfonamides:
- Crystaluria causing kidney damage.
- Kernicterus (Bil rubin induced brain dysfunction) in foetus and neonates.
- Hypersensitivity reactions.
- Aplastic anemia.
- Stevenson Johnson Syndrome.