What is Tuberculosis?
- Tuberculosis is an infectious disease caused due to bacteria of Mycobacterium species e.g.
- Mycobacterium tuberculosis. (Humans).
- Mycobacterium bovis. (animals).
- Mycobacterium avium complex. (Extra pulmonary tuberculosis).
- Usually tuberculosis affects respiratory system as it spreads through air.
- It attacks apex part of lungs and starts to grow there causing necrosis of affected area.
- The affected area is called as “tubercle” becomes radio-opaque during X-Ray examinations.
- Due to caused infection, the destructed alveoli tissues shows inflammation and cause irritation leading to cough (most common symptom) followed with hyperpyrexia.
What are symptoms of Tuberculosis?
- Persistent cough for three or more weeks.
- Hyperpyrexia at evening which disappears at night following profuse sweating.
- Blood in sputum.
- Excessive phlegm production.
- Weakness with chest pain.
- Weight loss.
Diagnosis of Tuberculosis:
- Monteux Test.
- Sputum Test.
- chest X-Ray examination.
Why Tuberculosis bacteria is tough to treat and produce resistance to most of available drugs?
- The Mycobacterium tuberculosis has a specific cell wall which contains very high amount of lipids and hence resists alcohols, alkalies, acids and even some disinfectants, which helps it to spread.
- The bacteria produces necrosis in surrounding area making entry of blood closed in affected area.
- The bacteria grows very slowly.
- The bacteria not only survives but also grows even when swallowed by WBCs.
Treatment of Tuberculosis:
Antitubercular Drugs:
- The drugs which are used in treatment of tuberculosis are called as antitubercular drugs.
- As tuberculosis bacteria is infamous for producing drug resistance the treatment is usually given in combination of drugs avoiding use of single drug.
- The drugs used in the treatment are classified on the basis of their toxicity as first line drugs, second line of drugs and third line drugs.
First Line Drugs:
- Isoniazid.
- Ethambutol..
- Pyrazinamide.
- Streptomycin.
- Rifampicin.
Second line Drugs:
- Para Amino Salicylic Acid.
- Cycloserine.
- Kanamycin.
- Amikacin.
- levofloxacin
Third line Drugs:
- Clarithromycin.
- Rifabutin.
- Linezolide.
Classification of Antitubercular Drugs Based on Chemical Structure:
- Salicylic Acid Derivative:
- e.g. Para Amino Salicylic Acid.
- Pyridine Derivatives:
- Isoniazid.
- Ethionamide.
- Prothionamide.
- Pyrazine Derivative:
- Pyrazinamide.
- Ehtylenediaminobutanol Derivatives.
- Ethambutol..
- Antibiotics:
- Streptomycin.
- Rifampicin.
- Kanamycin.
- Miscellaneous:
- Fluroquinolones : Moxifloxacin, Levofloxacin.
- Macrolides: Sparfloxacin.
- Tetracyclines: Minocycline
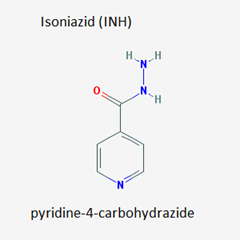
Isoniazid:
- It is a first line compound used in treatment of tuberculosis.
- It is also known as INH, Isonicotinic acid hydrazide.
- This drug is a simple pyridine derivative.
Mechanism of action:
- Isoniazid is in fact a “prodrug” as after bacterial metabolism it gets converted to active compounds like,
- Isonicotinicaldehyde.
- Isonicotinic acid.
- Isonicotinamide.
- It is supposed to act by interfering with functions of bacterial enzymes.
- However, its main mechanism of action is supposed to be the “Mycolic acid” synthesis inhibition.
- Mycolic acid is one of the major ingredients of the mycobacterial cell wall, inhibiting its synthesis INH makes the Mycobacterium vulnerable causing its death.
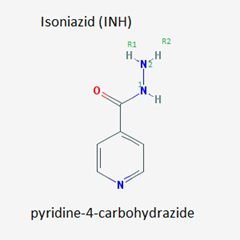
SAR of Isoniazid:
- The N1 in hydrazine side chain is essential for action and hence should not be substituted.
- The N2 can be substituted with alkyl compounds to get active products.
- The Pyridine ring is found essential for activity, substitution results into loss of activity.
- Many analogues which were developed are not found superior to the parent drug.
- However, the R2 replacement with isopropyl group resulted in formation of a new drug “Iproniazid” possessing “psychomotor stimulant activity.
Metabolism & Excretion:
- Isoniazid is get metabolized by “acetylation”.
- It get excreted freely in urine.
Adverse Effects:
- Neuritis.
- Hepatotoxicity.
- Anorexia.
- vomiting.
- Nephrotoxicity.
Dose:
- 50mg to 300mg daily in combination with other antitubercular drugs.
- Maximum allowed dose is 900mg/day.
- Vit. B6 (Pyridoxine) is usually given with INH to reduce neuritis.
Ethambutol.
- Ethambutol is abbreviated as “E” or “EMB”.
- It is a water soluble bacteriostatic drug which shows a great absorption when taken orally (80%).
- Chemically it is a aliphatic diamine with two butanol sidechains.
SAR of Ethambutol:
- Presence of two amino moieties is essential for action.
- Presence of branched alkyl groups on the nitrogens of amino group also influences the activity.
- Addition of heteroatom to ethylene moiety diminishes the activity.
- Replacement of alcoholic –OH groups with –OCH3 (methoxy) or –OC2H5 (ethoxy) groups produced equipotent compounds.
Mechanism of Action:
- Ethambutol works by interfering with cell wall formation of Mycobacterium and hence produce bacteriostatic action in multiplying bacterias.
“Mycolic acid is one of the main constituent of mycobacterial cell wall, Ethambutol prevents formation of “Mycolyl-Arabinogalactan-Peptidoglycan” complex.
By preventing synthesis of arabinogalactan from arabinosyl transferase.”
Metabolism:
- Metabolism of Ethambutol takes place in liver and 75% of the drug gets excreted unchanged.
Adverse / Toxic Effect:
- Optic Neuritis.
- Nausea.
- Anorexia.
- Hyperuricaemia.
Therapeutic uses:
- In treatment of pulmonary and extra pulmonary tuberculosis.
- As first line drug in treatment of Tb.
Rifampicin.
- Rifampicin is an antibiotic belonging to the class “Macrocyclic Antibiotics: Rifamycins”.
- They were obtained from soil bacterium “Streptomyces mediterranei”.
- Total “7” rifamycins being isolated as Rifamycin A,B,C,D,E,S,SV.
Mechanism of Action:
- Rifampicin inhibits bacterial “DNA dependent RNA polymerase” enzyme causing inhibition of RNA synthesis and in turn inhibition of protein synthesis.
- Rifampicin is highly effective is its MIC (Minimum Inhibitory Concentration) is just 0.005 to 0.2 microgram per ml.
SAR of Rifampicin:
- The presence of flat naphthalene ring is essential for antibacterial action.
- Presence of –OH groups and double bonds are essential for action.
- Intact macrocyclic ring is essential for action.
- Presence of carbonyl group is also found essential for action.
- Its a zwitterion and having good lipid as well as water solubility.
Therapeutic Uses:
- As first line therapy in treatment of Tuberculosis in combination with other drugs.
- Also used in treatment of Leprosy.
- Along with Doxycycline it is used to treat Brucellosis.
- In prophylaxis of H. meningitides.
Side effects/ Adverse effects:
- Change in urine color.
- Hyperbilirubinaemia.
- Thrombocytopenia.
- Hepatotoxicity.
- Rashes.
Pyrazinamide
- It is a “Pyrazine” derivative classified in first line drugs for treatment of Tuberculosis.
Mechanism of Action:
- Exact mechanism of action is yet not known.
- It gets converted to “pyrazinoic acid” in bacteria which is its active form.
- It is known to disrupt bacterial cell membrane and transport mechanisms.
SAR of Pyrazinamide:
- The replacement of pyrazine ring with any other ring diminishes the activity.
- The monosubstituted ring is essential as di substituted rings found to have lesser activity, however, after introduction of QSAR to di substituted derivatives were introduced which were found to have activity.
Therapeutic uses:
- It is used in treatment of Tuberculosis and classified as first line of drugs.
- It is used in combination with INH and rifampicin in cases where resistance is suspected.
Side effects / Adverse effects:
- Hepatotoxicity.
- Hyperuricaemia.
- Arthralgia.
- GI upset.
Streptomycin.
- It is an aminoglycoside antibiotic.
Mechanism of Action:
- Streptomycin irreversibly inhibits bacterial protein synthesis and is bactericidal.
- Streptomycin binds with 30S unit of bacterial ribosome and causes inhibition of bacterial synthesis in the following ways,
- Interferes with initiation complex of peptide formation.
- Misreading of mRNA which leads to formation of nonfunctioning or toxic proteins.
- Breaking of Polysomes into nonfunctional monosomes.
Uses of Streptomycin:
- As first line drug in treatment of Tuberculosis in combination with other drugs.
- In treatment of septicemia in combination with penicillin or metronidazole.
- In treatment of tularemia and plague.
- In treatment of streptococcal infections along with penicllins.
Side effects/ Adverse effects:
- Hepatotoxicity.
- Nephrotoxicity.
- Ototoxicity.
- GI upset.
Note:
- Although it is used with penicillins many times both drugs must not be mixed physically as they cause neutralization of each others activity.